These chemicals present on the tip of the matchstick. The role of phosphorous.
 The Striking Science Of Match Sticks Science And Learning Outdoors
The Striking Science Of Match Sticks Science And Learning Outdoors
The glass in the match creates friction with the glass in the safety paper which ignites the head and then burns the wood.

Composition of matchstick head. It is easily ignited by the heat of friction against a rough surface. The heads of strike anywhere matches are composed of two parts the tip and the base. The match heads are composed of sulfur this is what you smell mixed with oxidizing agents like potassium chlorate colorants dyes fillers glue binders starch and powdered glass.
The chemical present in matchstick is potassium chlorate sulfur starch and glue. Phosphorous sulfide is the chemical compound that ignites match heads. According to the use and the composition of the medicine head matchsticks can be divided into two categories.
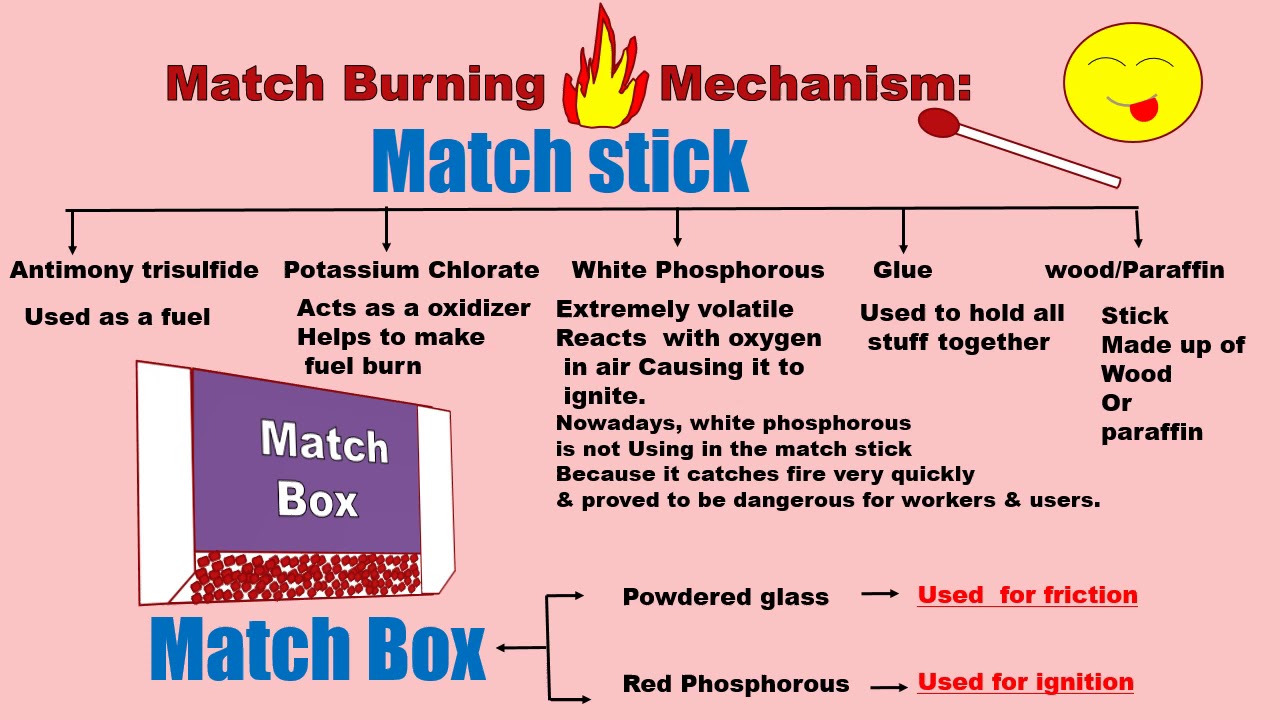
Matchbox striking surface contains red phosphorus powdered glass and glue. Wooden matches are packaged in matchboxes and paper matches are partially cut into rows and stapled into matchbooks. These chemicals present on the tip of the matchstick.
Matches ignite due to the extreme reactivity of phosphorus and chlorate mix in the match head. A match is a tool for starting a fire typically matches are made of small wooden sticks or stiff paper one end is coated with a material that can be ignited by frictional heat generated by striking the match against a suitable surface. According to the shape of the package.
Daily matches common matches and special matches. The tip contains a mixture of phosphorus sesquisulfide and potassium chlorate. It s found in the heads of strike anywhere matches and in the strip on the side of safety match boxes.
Other ingredients of match heads include potassium chlorate phosphorous sesquisulfide sulfur glass powder binders and fillers. Hope it helps you. These can include antimony iii sulfide and or sulfur added as fuel to help the match head burn.
Matchsticks is a mixture of antimony trisulphide potassium chlorate white phosphorus with some glue and starch is applied on the head of the match made of suitable wood. The match head contains an oxidising agent commonly potassium chlorate and glue to bind it to further abrasive materials and other additive compounds. Phosphorus sesquisulfide is a highly reactive non toxic chemical used in place of white phosphorus.
 Henrymz S Blog Chemistry Of Matches
Henrymz S Blog Chemistry Of Matches
How Match Is Made Material Manufacture Making History Used Parts Composition Product Machine History
 The Chemistry Of Matches Compound Interest
The Chemistry Of Matches Compound Interest
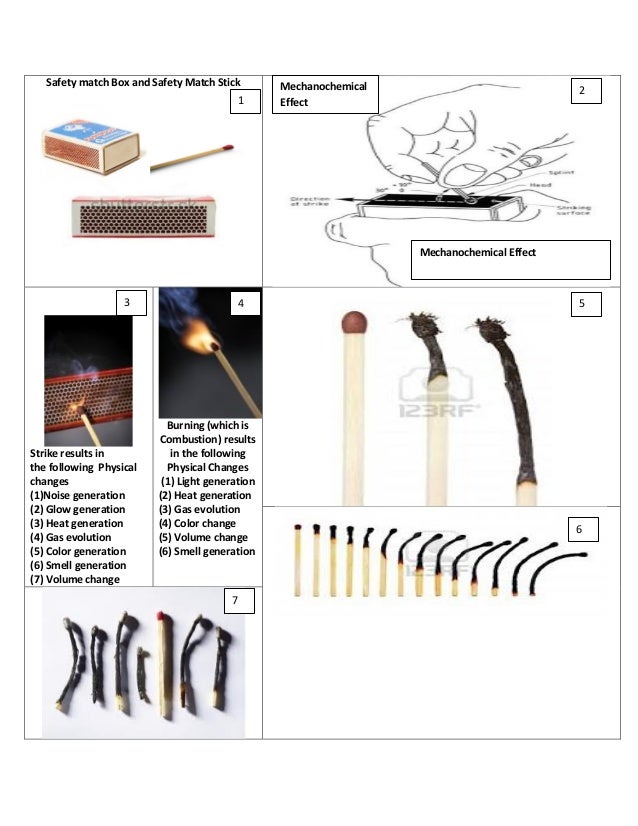 Physical And Chemical Changes During The Burning Of A Safety Match
Physical And Chemical Changes During The Burning Of A Safety Match

 Matchstick Heads High Resolution Stock Photography And Images Alamy
Matchstick Heads High Resolution Stock Photography And Images Alamy
 What Is The Composition Of The Head Of A Matchstick Brainly In
What Is The Composition Of The Head Of A Matchstick Brainly In
 Auto Ignite Match Stick Applied Chemistry Science Forums
Auto Ignite Match Stick Applied Chemistry Science Forums
 How Do Safety Matches Work Science Abc
How Do Safety Matches Work Science Abc
 Chemistry Of Matches How Do Matches Work Chemical Used In Match Heads Youtube Youtube
Chemistry Of Matches How Do Matches Work Chemical Used In Match Heads Youtube Youtube
 Infographics Matches Chemical Composition The Combustion Temperature Stock Photo Picture And Royalty Free Image Image 41609867
Infographics Matches Chemical Composition The Combustion Temperature Stock Photo Picture And Royalty Free Image Image 41609867

